To understand "What is life?", we first examine the function of each biomolecule in biological processes. Our laboratory focuses on chemically synthesizing glycans to clarify their molecular roles and explore medical applications.
Why sugar chains? Want to unlock the immeasurable potential of glycans.
All vertebrate cells are coated with glycans, which play vital roles in protection, communication, and substance exchange at the cell membrane. Alongside nucleic acids and proteins, glycans are known as the "third life chain," though their molecular basis of the biological functions remain largely unexplored. Understanding the glycan’s functions could lead to new treatments for major diseases, including cancer, infections, immune disorders, and Alzheimer's disease.
Obstacles to Glycan Research = Diversity and Scarcity
Unfortunately, research on glycans, the "third life chain", lags behind that on nucleic acids and proteins.
This is because of two reasons.
- Carbohydrates in living organisms exhibit vast structural diversity.
- Glycans are scarce in living organisms.
Research progress is hindered by limited access to sugar chains, despite their importance. Still, Japanese researchers remain at the forefront of this field. Once these glycans become readily accessible, significant advancements are expected.
So we want to artificially create glycans at will! Organic synthesis comes first!
To overcome barriers in glycan research, it's essential to produce many pure glycans for study. Organic synthesis is useful, but further research is needed to replicate entire glycans in the body.
That is why our laboratory has been tirelessly accumulating research and continuing to refine the production technology.
As a result, we have succeeded in the synthesis of many sugar chains that had been said to be impossible so far.
The beginning is "creation" = creation → elucidation → application
Advancements in synthesis technology have enabled the production of glycans that were previously unattainable. This progress allows researchers to investigate the functions of these glycans, which could not be studied by many collaborators before. As previously unknown functions become elucidated, the potential for applied research increases, ultimately facilitating the development of medicines based on the specific properties of these glycans.
The world of glycans shifts as our production technology advances—and so does our perspective on life.
Let's master glycan synthesis and change the world of glycans!
- A new way to create sugar chains
- Synthesizing complex glycans not yet made chemically
- Producing functional glycans to elucidate the function of glycans
- Making smart glycans for pharmaceutical applications
Anyone who is motivated is welcome to join us!
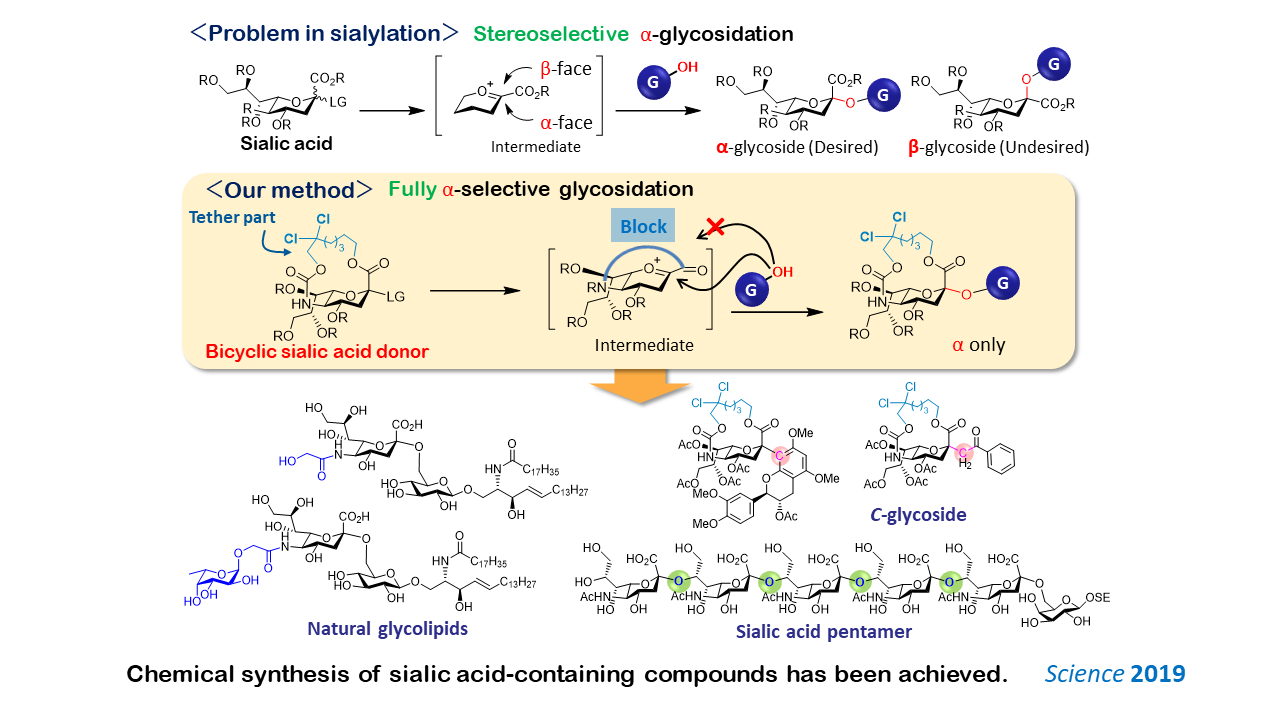
1-1. Development of an α-Selective Sialylation Method
Sialic acid-containing glycans on the cell surface play an important role in cell-cell communication. Conversely, sialic acid is an important target for drug development because bacteria and viruses bind to the sialic acid-containing glycans. Sialic acid binds to other molecules through two types of linkages: α- and β-linkages. However, only α-linked sialic acid exists in nature. The chemical synthesis of α-sialosides has been a challenge in carbohydrate chemistry for 50 years. We have first achieved a fully stereoselective α-glycosidation of sialic acid by using a bicyclic donor, in which the β-face of sialic acid is blocked by the tether moiety. This method enables the synthesis of sialic acid linked not only to an oxygen atom (O-glycoside) but also to a carbon atom (C-glycoside), which is useful for vaccine development. Furthermore, we have achieved the chemical synthesis of glycolipids and sialic acid oligomers, which are difficult to synthesize with conventional methods.
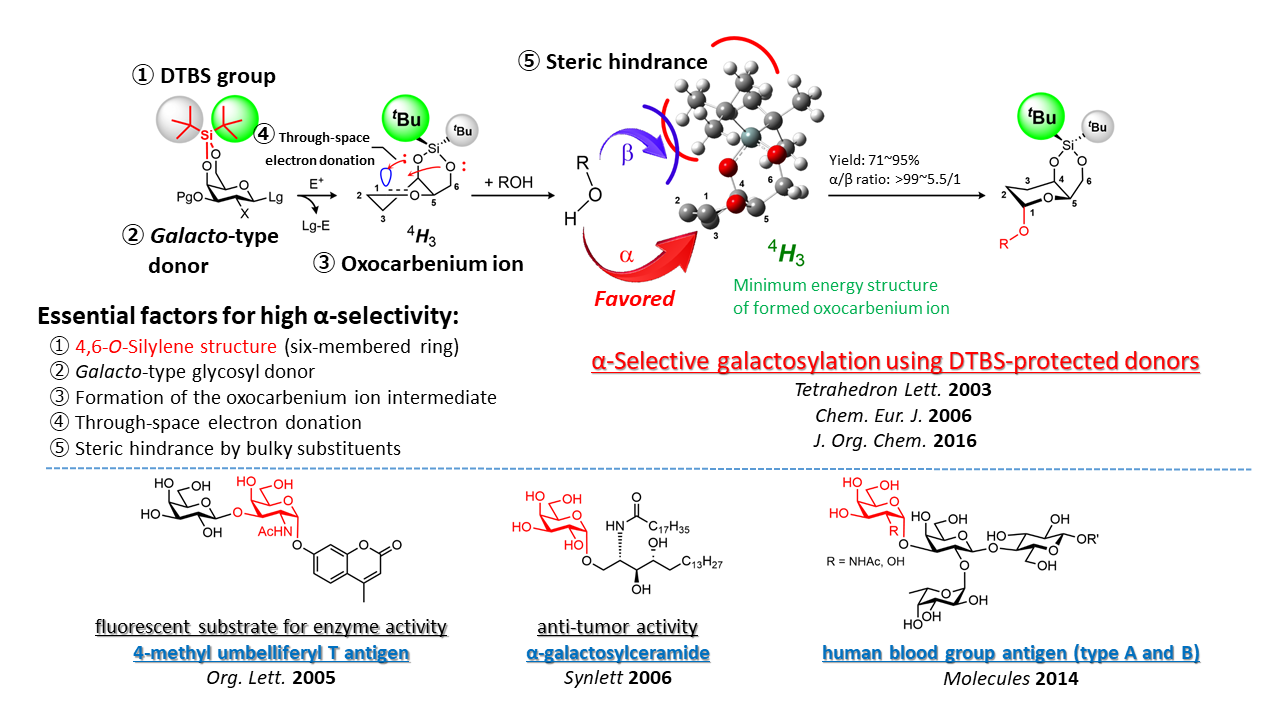
1-2. Development of an α-Selective Galactosylation Method Using the DTBS Effect
Precise control over the stereochemistry at the anomeric position (α and β) is essential for the chemical synthesis of glycans. To achieve this, highly stereoselective glycosylation reactions are required. However, α(1,2-cis)-selective glycosylation remains one of the most challenging issues in glycochemistry, as no broadly applicable and reliable methodology has been fully established. To address this, we designed a galactosyl donor bearing a di-tert-butylsilylene (DTBS) group at the C4- and C6-positions. Glycosylation using this donor afforded α-galactosides in high yield and excellent stereoselectivity–a phenomenon referred to as the “DTBS effect”. Using this original approach, we have successfully constructed α-galactose-containing structures in a variety of bioactive glycans. Furthermore, to elucidate the detailed reaction mechanism, we are combining experimental techniques with computational chemistry in collaborative research.
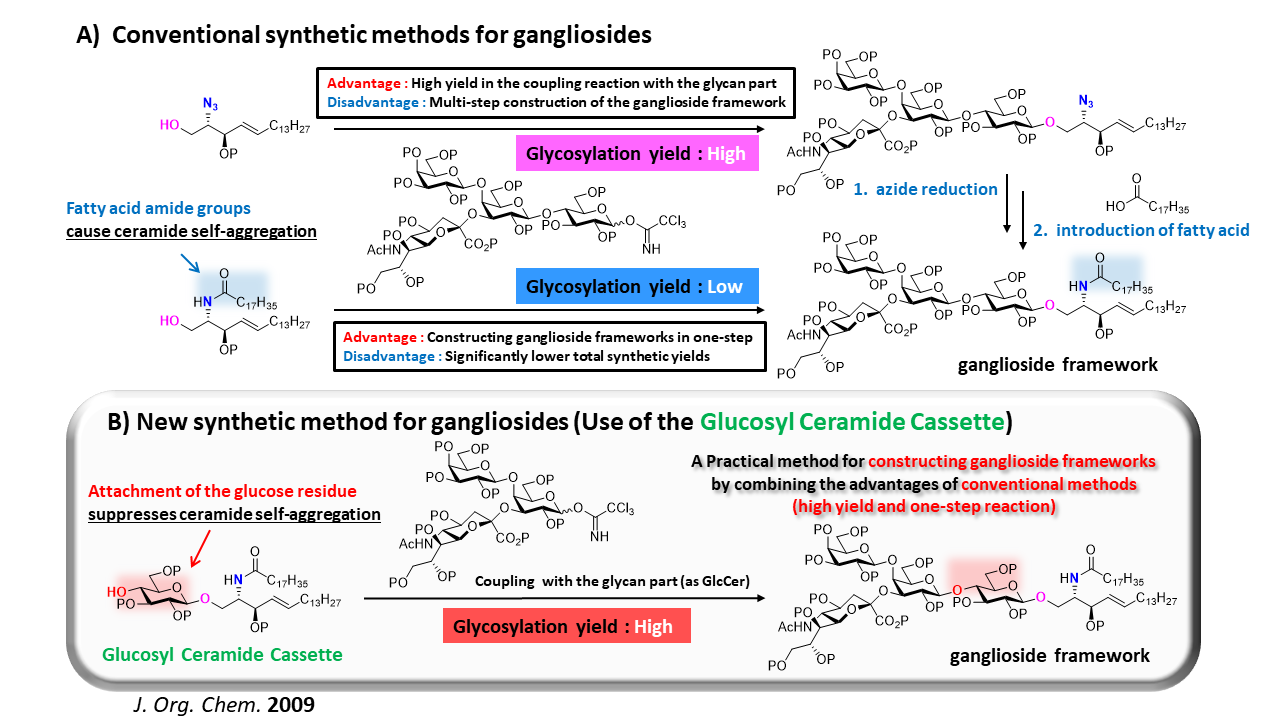
1-3. Development of an Efficient Ganglioside Synthesis Method: The Glucosyl Ceramide Cassette Approach
Our laboratory has achieved the total synthesis of bioactive glycosphingolipids known as gangliosides, which consist of glycan chains containing sialic acid linked to ceramide moieties. However, the original synthetic route required multiple reaction steps to introduce the ceramide moieties after glycan construction. This often resulted in decomposition of the carefully assembled glycan during the final stages of total synthesis, leading to low overall efficiency (Fig. A, top). Alternatively, a direct coupling strategy between the glycan and ceramide moieties was possible. However, due to the unique chemical structure of ceramides, which promotes self-aggregation via hydrogen bonding and hydrophobic interactions, glycosylation yields were extremely poor (Fig. A, bottom). To overcome this issue, we developed a glucosylceramide cassette, in which a glucose unit (corresponding to the reducing end of the ganglioside) is pre-attached to the ceramide moiety to suppress self-aggregation (Fig. B). Using this cassette approach, ganglioside frameworks can now be assembled in a single step and with high yield by the coupling of the non-reducing end glycan part (except for the terminal glucose residue) and the preformed glucosylceramide.
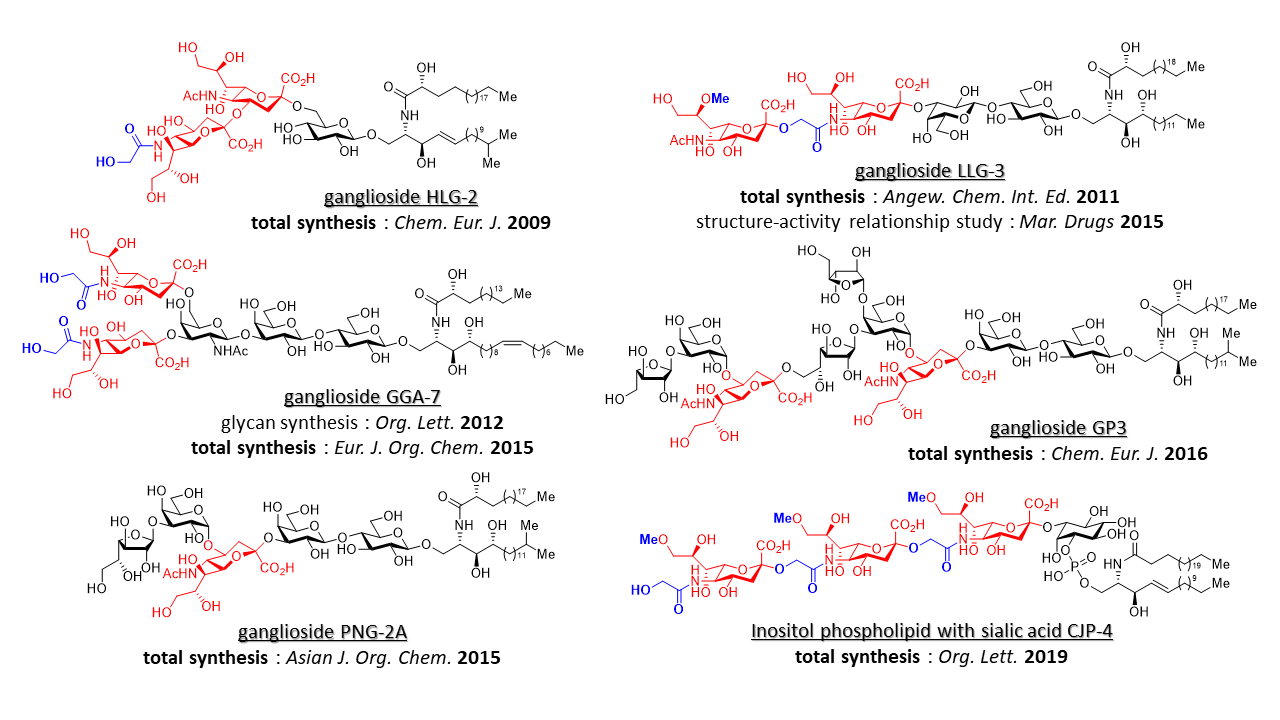
2. Natural Product Synthesis: Total Synthesis of Sialic Acid-Containing Glycolipids from Echinoderms
Sialic acid-containing glycolipids isolated from echinoderms, such as sea cucumber and starfish, have been reported to exhibit potent neurite outgrowth activity. Unlike mammalian gangliosides, these glycolipids feature internal sialic acid residues and unique chemical modifications including N-glycolylation and O-methylation, which makes them intriguing not only from a biological perspective but also as synthetic targets. In response to this, we undertook the total synthesis of echinoderm-derived sialic acid-containing glycolipids using glycosylation and synthetic methodologies originally developed in our laboratory. As a result, we have successfully achieved the total synthesis of the following compounds.
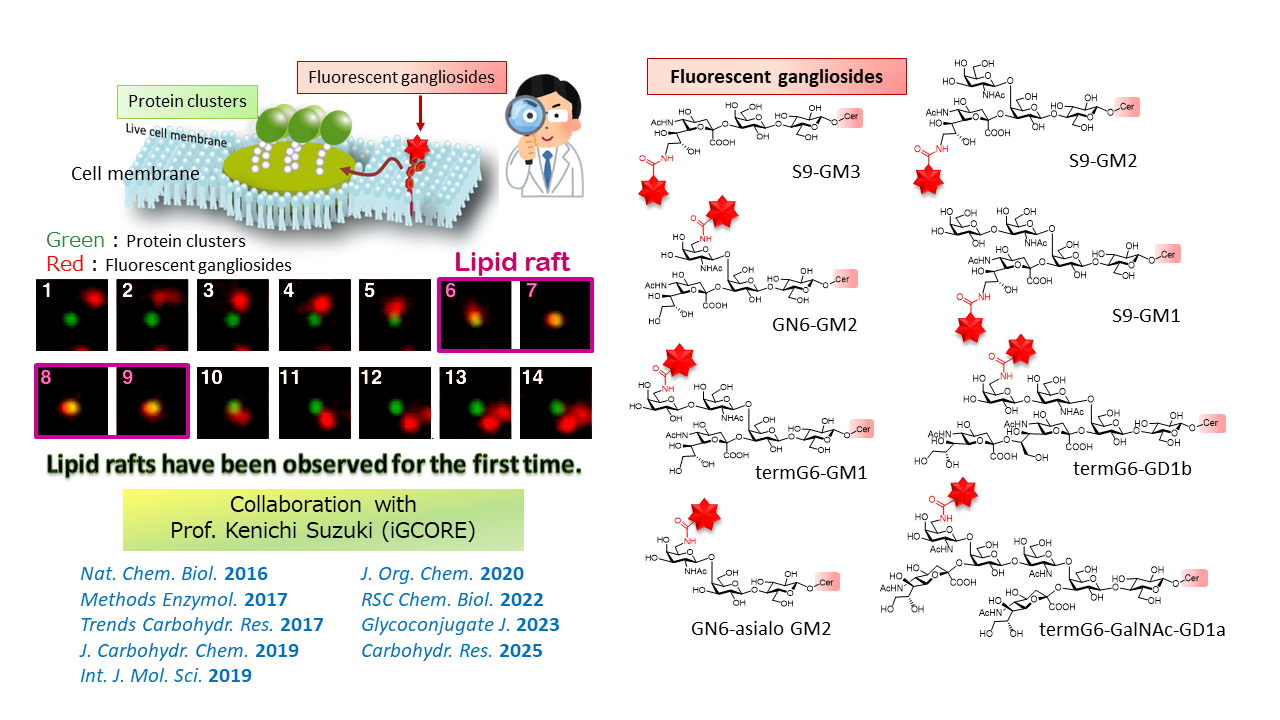
3. Ganglioside Probes Enabled Chemical Biology of Cell Membranes
Glycosphingolipids (gangliosides) play an important role in signal transduction by forming membrane domains (lipid rafts) with protein receptors and cholesterol on the cell membrane. However, the existence of lipid rafts has never been demonstrated. Direct observation of lipid rafts has been required to solve this problem. We have developed a fluorescently-labeled gangliosides, which enabled observation of gangliosides on living cells (single molecule imaging). Single molecule imaging of fluorescent gangliosides enabled the observation of the lipid raft formations, thus demonstrating their existence for the first time. (Collaborative research with Prof. Kenichi Suzuki in iGCORE)
We have developed more than 30 fluorescent gangliosides by our original synthetic methods. These gangliosides were used for the observation of lipid raft formations and interactions between gangliosides and protein receptors. Lipid rafts are deeply associated with signal transduction and important infections and diseases, such as influenza and cancer. Therefore, our research will contribute to the elucidation of the mechanism of these diseases and drug development.
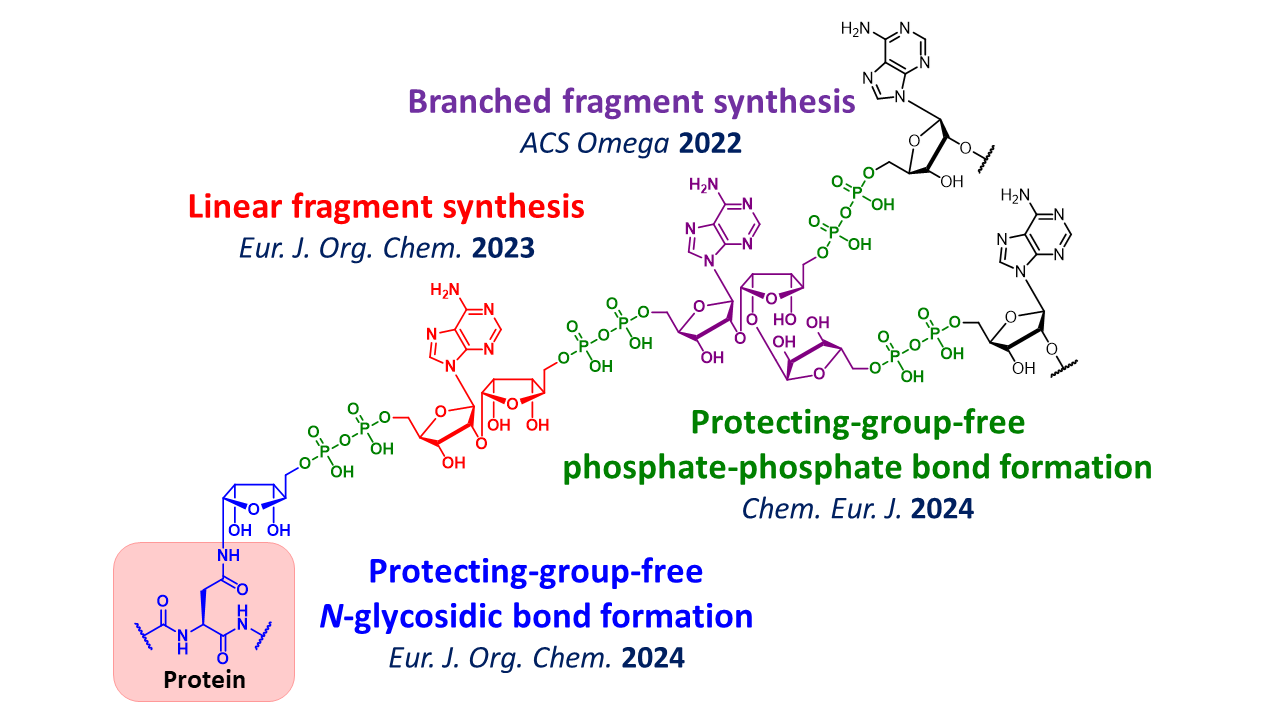
4. Development of Synthetic Methods for ADP-ribose Chains
ADP-ribose chains are biomolecules that, like glycans, are attached to proteins as a form of post-translational modification and play a crucial role in DNA damage repair. In addition to their biological significance, they possess structural features of both glycans and nucleic acids, making them highly attractive synthetic targets. To achieve the controlled synthesis of ADP-ribose chains, we have established synthetic methods for linear and branched fragments based on techniques in carbohydrate chemistry. Furthermore, we have developed novel methods to efficiently form the phosphate-phosphate bond and the reducing-end N-glycosidic bond found in ADP-ribose chains. By using these synthetic methods, we are advancing ADP-ribose chain chemical biology research.