Development of Z-selective Horner-Wadsworth-Emmons reagents
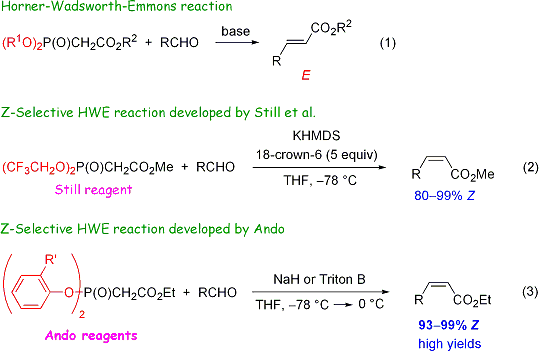
Horner-Wadsworth-Emmons (HWE) reaction (scheme 1) is a versatile reaction, which affords C=C bond-containing molecules, such as a,b-unsaturated esters, with high E-selectivity. On the other hand, Still's method (scheme 2) affords the products with high Z-selectivity and has been the most frequently used Z-selective HWE reaction.1 However, this method requires strictly anhydrous conditions due to moisture sensitive KHMDS. In addition, as much as 5 equivalents of relatively expensive and hygroscopic 18-crown-6 are necessary. Under these circumstances, we developed a more practical Z-selective HWE reaction that uses diarylphosphonoacetate derivatives.2,3,4 The new Z-selective HWE reaction does not require strictly anhydrous conditions and affords the desired a,b-unsaturated esters by using only inexpensive reagents and starting materials (scheme 3). This practical method has been used for the synthesis of a variety of compounds including total syntheses of biologically active compounds. Some of these Z-selective HWE reagents are commercially available from TCI Co., Ltd. (TCI products info)
1) Still, W. C.; Gennari, C. Tetrahedron Lett. 1983, 24, 4405-4408.
2) Ando, K. Tetrahedron Lett. 1995, 36, 4105-4108.
3) Ando, K. J. Org. Chem. 1997, 62, 1934-1939.
4) Ando, K. J. Synth. Org. Chem., Jpn. 2000, 58, 869-876. (written in Japanese)
