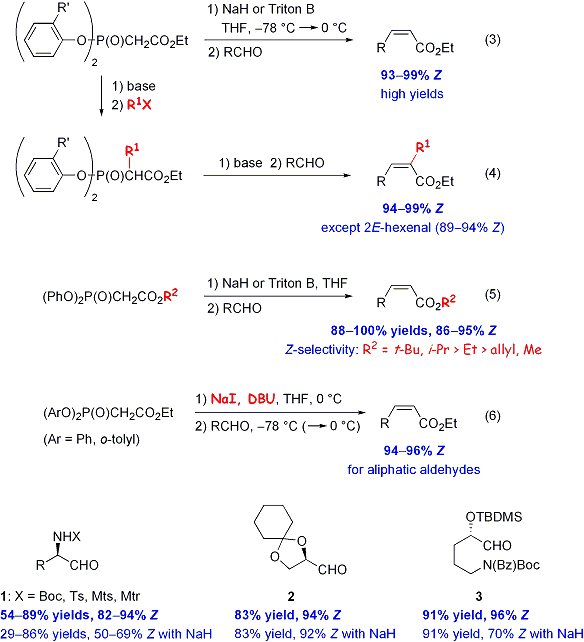
Synthesis of a,b-unsaturated esters by Z-selective HWE reactions
These HWE reagents can be alkylated at the a-position. The resultant compounds afford a-alkyl-a,b-unsaturated esters with excellent Z-selectivity (scheme 4).1
In these reactions, the HWE reagents with a large ester group generally afforded the products with higher Z-selectivity than those with a small ester group (scheme 5).2
HWE reactions generally require a strong base, such as NaH, whereas Masamune, Roush and co-workers have reported that the reactions were successfully promoted by relatively weak bases, such as DBU and i-Pr2NEt, in the presence of Li+. This can be attributed to the chelating of the P=O and C=O of the HWE reagents with Li+, which would increase the acidity of the a-proton of the HWE reagents. With this in mind, we sought to develop a Z-selective HWE reaction promoted by a relatively weak base and found that the use of DBU and NaI afforded the desired a,b-unsaturated esters with high Z-selectivity (scheme 6).4 Under these conditions, aldehydes having a nitrogen or oxygen at the a-position (e.g. 1-3) can be used to synthesize the corresponding a,b-unsaturated esters without side reactions, such as racemization. In the case of 1 and 3, the Z-selectivity was greatly improved compared to the reactions promoted by NaH.
1) Ando, K. J. Org. Chem. 1998, 63, 8411-8416.
2) Ando, K. J. Org. Chem. 1999, 64, 8406-8408.
3) Blanchette, M. A.; Choy, W.; Davis, J. T.; Essenfeld, A. P.; Masamune, S.; Roush, W. R.; Sakai, T. Tetrahedron Lett. 1984, 25, 2183-2186.
4) Ando, K.; Oishi, T.; Hirama, M.; Ohno, H.; Ibuka, T. J. Org. Chem. 2000, 65, 4745-4749.
