Development of α-selective ribofuranosylation reaction
Ribofuranosides are found in a range of biologically active natural products, some of which are potentially useful as cosmetics, food additives, and medicines. Ribofuranosides have two stereoisomers, a- and b-ribofuranosides, and the stereoselective synthesis of these isomers is necessary. While the synthesis of b-isomers is generally easy, it is still difficult to synthesize a-ribofuranosides completely stereoselectively. Methods in the literature often provide a-ribofuranosides containing a small amounts of b-isomers. In addition, many of the current methods require toxic heavy metals and explosive reagents to achieve high a-selectivity, making these methods inapplicable to the synthesis of products which we take into our bodies.
To solve these problems, we developed a novel a-selective ribofuranosylation of alcohols using a ribofuranosyl iodide 1 as a glycosyl donor. Ribofuranosyl iodides are so reactive that they do not require activating reagents such as heavy metals to react with alcohols. In addition, we found that the a-selectivity was enhanced up to >99:1 by the addition of triphenylphosphine oxide. This novel a-selective ribofuranosylation reaction would solve the two disadvantages which the current methods have. In particular, the use of toxic reagents can be circumvented, making this new method suitable for the production of cosmetics, food additives, and medicines1.
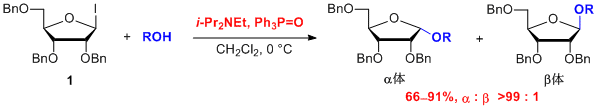
1) Oka, N.; Kajino, R.; Takeuchi, K.; Nagakawa, H.; Ando, K. J. Org. Chem. 2014, 79, 7656–7664.
