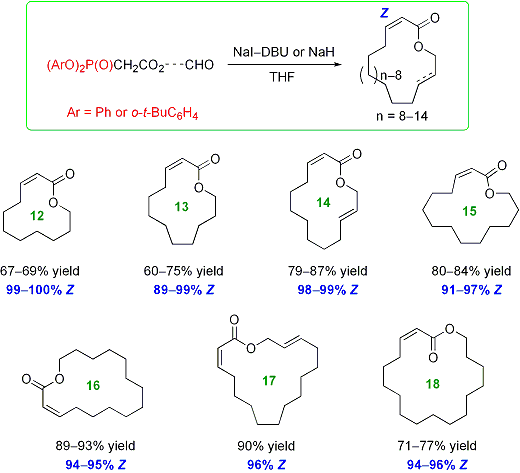
Synthesis of a,b-unsaturated macrolactones by Z-selective intramolecular HWE reactions
Development of an efficient method to synthesize Z-a,b-unsaturated macrolactones is crucial for the synthesis of many bioactive macrolides. We studied the synthesis of Z-a,b-unsaturated macrolactones via intramolecular Horner-Wadsworth-Emmons reaction and found that diaryl phosphonate reagents (ArO)2P(O)CH2CO2---CHO afforded the corresponding 12 to 18-membered a,b-unsaturated macrolactones shown below in a highly Z-selective manner, while the diethyl phosphonate counterparts gave the same macrolactones E-selectively (52-82% yield, 89-99% E).
Ando, K.; Narumiya, K.; Takada, H.; Teruya, T. Org. Lett. 2010, 12, 1460-1463.
