Synthesis of nucleosides phosphorylated at their carbonyl groups
In the biosynthetic pathways of nucleic acids, there are several reactions in which the carbonyl group of a nucleobase is activated by phosphorylation and replaced by an amino group or a sulfur atom. We developed a method for the synthesis of carbonyl-phosphorylated inosine, an active intermediate analog, to elucidate the reaction mechanism of the biosynthesis of adenylosuccinate. Such molecules would also be useful to develop enzyme-responsive molecules. Although the phosphorylation of nucleoside carbonyl groups is difficult to achieve using existing methods, we have succeeded in the synthesis of O6-phosphorylinosines by using CMPT or CMMT, which are acidic activators with low nucleophilicity, to suppress the degradation of unstable synthetic intermediates.
In addition, we succeeded in synthesizing an O2-phosphorylxanthosine derivative as a simple model compound of adenosine monophosphorylated xanthosine monophosphate (AMP-XMP), which is known as a biosynthetic intermediate of guanosine monophosphate, and clarified its hydrolytic stability.
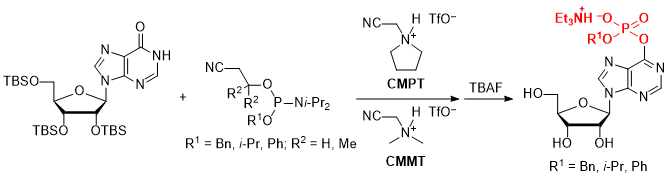
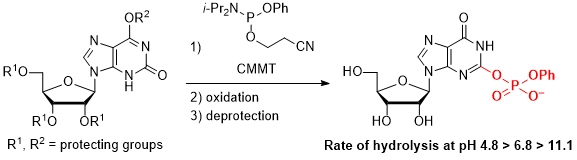
1) Oka, N.; Morita, Y.; Itakura, Y.; Ando, K. Chem. Commun. 2013, 49, 11503-11505.
2) Oka, N.; Hirabayashi, H.; Kumada, K.; Ando, K. Bioorg. Med. Chem. Lett. 2021, in press.
