Stereoselective synthesis of 1,2-cis-glycosyl sulfones and their application
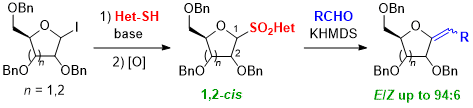
Glycosyl sulfones are useful as synthetic intermediates for various sugar derivatives and as biologically active compounds. However, most of the previous research has been conducted using easily synthesized 1,2-trans-isomers, and little progress has been made in the study of 1,2-cis-isomers. We developed a novel method for the synthesis of 1,2-cis-glycosyl sulfones using glycosyl iodides as glycosyl donors. The resulting 1,2-cis-glycosyl sulfones were useful as Julia-Kocienski reagents to give exo-glycals in good yields from aldehydes. The 1,2-cis-glycosyl sulfones derived from ribose and glucose afforded the corresponding exo-glycals with higher E-selectivity than the 1,2-trans-isomers, and the difference was particularly large for the ribose derivatives. Thus, our study has revealed the usefulness of 1,2-cis-glycosyl sulfones. The difference between the 1,2-cis- and 1,2-trans-isomers in the Julia-Kocienski reaction has also been demonstrated.
1) Oka, N.; Mori, A.; Ando, K. Eur. J. Org. Chem. 2018, 6355-6362.
2) Oka, N.; Mori, A.; Suzuki, K.; Ando, K. J. Org. Chem. 2021, 86, 657-673.
3) Oka, N.; Suzuki, A.; Mori, A.; Ando, K. Eur. J. Org. Chem. 2021, 5922-5933.
