Research Topics
- Syntheses of novel heterocyclic compounds
- Preparation of selenating reagents
- Biological activities of the novel heterocyclic compounds
- Syntheses of stereoselective glycosides of ulosonic acids
■ Syntheses of novel heterocyclic compounds
- Preparation of heterocyclic compounds using primary selenoamides
- The preparation of 1,3-selenazines and 1,3-thiazines
(1,3-selenazines were investigated regarding their biological activities) -
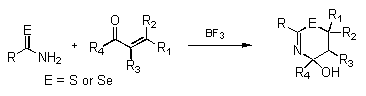
- The reactions with bisacyl chlorides
-
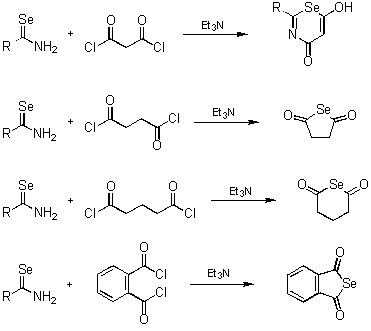
- The reactions with haloacyl halaides
-
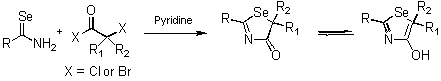
- Preparation of heterocyclic compounds using selenazadienes
- The reactions with dimethylacetylene dicarboxylate (DMAD)
-
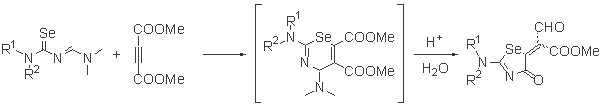
- The reactions with haloacyl halide
-
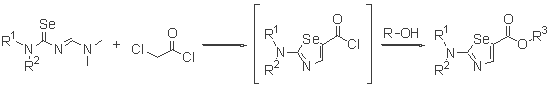
- Cyclization reaction using TiCl4
- Syntheses of 4,5-dihydroselenophenes
-
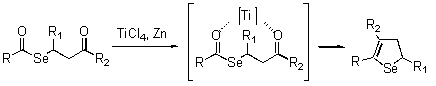
- Cyclization reaction of primary amines with selenoketene
- Syntheses of 5,6-dihydroselenines
-
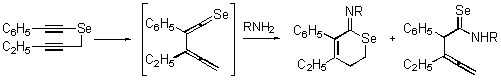
- Syntheses of b-selenolactams
-
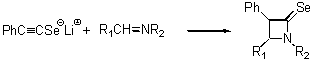
- Synthesis of 1,4-Oxaselenin
-
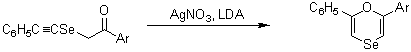
■ Preparation of selenating reagents
- Potassium selenocarbonic acid
- Preparation
-
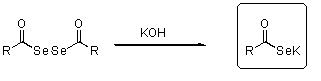
- Applications
-
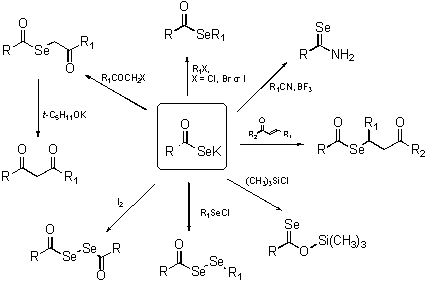
- LiAlHSeH
- Preparation
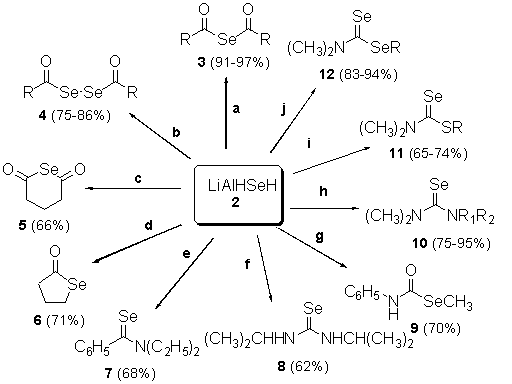
-
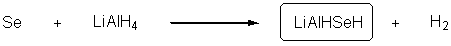
- Applications
-
■ Biological activities of the novel heterocyclic compounds
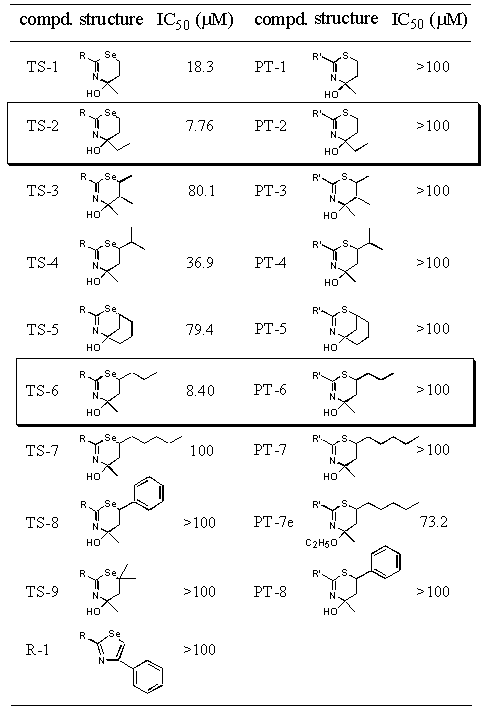
After HT-1080 cells (1 x 104 cells/well) seeded into the 96-well plate were preincubated for 48 hr, the culture was replaced by the medium containing various concentration (0.1 mM-100 mM) of compounds and incubated for another 24 hr. The survival cells were determined by crystal violet method. Each assay was performed in triplicate.
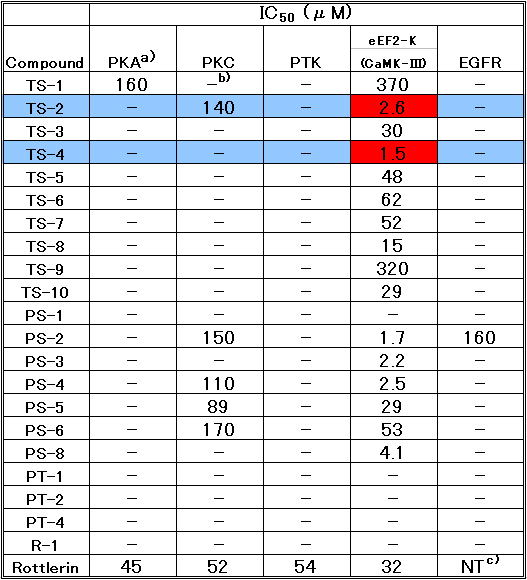
The compounds 4-ethyl-4-hydroxy-2-p-tolyl-5,6-dihydro-4H-1,3- selenazine (TS-2) and 4-hydroxy-4-methyl-6-propyl-2-p-tolyl- 5,6-dihydro-4H-1,3-selenazine (TS-6) exhibited the strongest inhibitory effect among 1,3-selenazine derivatives. Other selenazine derivatives showed no effect. To compare the effect between 5,6-dihydro-4H-1,3-selenazine and 5,6-dihydro-4H-1,3-thiazine in which the selenium atom was replaced with the sulfur atom, we prepared 2-phenyl-5,6-dihydro-4H-1,3-thiazines (PT series) and examined their cytotoxicity in vitro. The derivatives had no inhibitory effects except for the weak inhibition of PT-7e. Thus TS-2 and TS-6 of 5,6-dihydro-4H-1,3-selenazine structure, which possess a six-membered ring containing nitrogen and selenium atoms, indicated the strongest cytotoxicity against cancer cell.
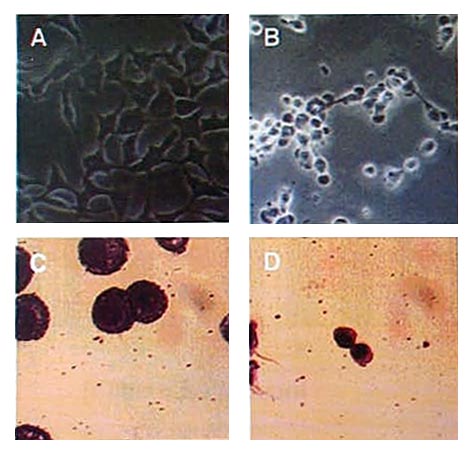
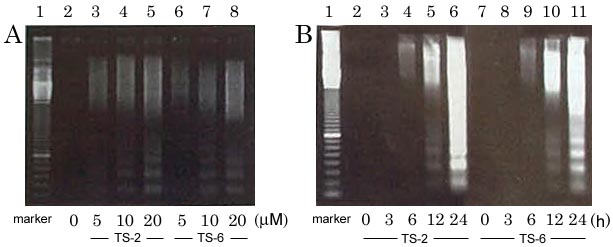
1,3-Selenazines induced dose-dependent DNA fragmentation in cancer cell, revealing a typical apoptosis characteristics.
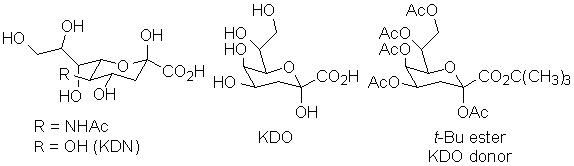
■ Syntheses of stereoselective glycosides of ulosonic acids
Ulosonic acids including N-acetylneuraminic acid (NANA), 3-deoxy-D- glycero-D-galacto-2-nonulosonic acid (KDN) and KDO (3-deoxy-D-mannno -2-octulosonic acid) are well known to form glycosides in the a -configuration in nature. A method was developed using samarium iodide under Barbier conditions for the synthesis of C-glycosides of ulosonic acids, NANA, KDN and KDO by the Prof. Linhardt's group (J. Am. Chem. Soc., 119, 1480, 1997; Tetrahedron Lett., 39, 5007, 1998.). NANA and KDN typically exist in a 2C5 conformation, thus, the reaction using samarium iodide exclusively affords the a -C-glycosides. In contrast, KDO exists in a 5C2 conformation, exclusively affording C-glycosides in the b -configuration (J. Am. Chem. Soc., 119, 1480, 1997; Tetrahedron Lett., 39, 5007, 1998; Carbohydr. Res., 308, 1998; J. Org. Chem., 64, 7254, 1999.).
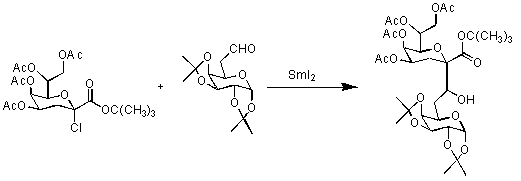
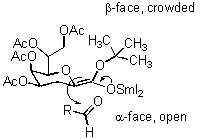
C-glycosides are catabolically stable analogues of important natural products and have been used as receptor ligands and glycosidase inhibitors. C-glycosides of ulosonic acids are of particular interest because of their potential pharmaceutical applications. KDO C-glycosides are also expected to have a conformation similar to the corresponding O-glycosides. A strategy was designed to stereoselectively synthesize the a -glycoside of KDO C-disaccharide through the reaction of t-butyl (4,5,7,8-tetra- O-acetyl-3-deoxy- a -D-manno-2-octulopyranosyl chloride)onate donor, with the 6-formylgalactopyranoside acceptor, in the presence of SmI2.