High hydrostatic pressure on protein systems
■Subjects
▼Enzymatic activity of oligomeric proteins
▼Refolding of proteins from their aggregates using high hydrostatic pressure
▼Study of amyloidosis using high-pressure electrophoresis
What is hydrostatic pressure?

Propagation of pressure in fluid is isotropic
Hydrostatic pressure is the pressure that is isotropically exerted by a fluid at equilibrium. For every 10 meters you go down under the sea, the pressure increases by 0.1 MPa. In thermodynamics, such pressurization can be converted to the chemical energy; under 200 MPa, water would be 4 times larger than that in ambient pressure, where water molecules would interact with solutes more tightly. In other words, pressure promotes hydration.
High hydrostatic pressure and biology

Pressure reversal of anaesthesia
Various bilological processes have been observed to be influenced by hygh hydrostatic pressure. One of the most famous phenomena is that high pressure in the range of several MPa causes anaesthetized animals to awake. This is called pressure reversal of anaesthesia.
Retardation of the microorganism growth by pressure is also observed. This phenomenon is utilized in food processing technology.
Pressure-hydration effect is also exerted on the biological macromolecules. This enables pressure to solubilize the denatured and aggregated proteins for their refolding.
High hydrostatic pressure as thermodynamic tweezers
Pressure on the protein complex
Hydrostatic pressure shifts the chemical equilibrium involving proteins, to modify the state of proteins, enzyme reaction rate, etc. Accordig to Le Chatleie's principle, pressure shift the equilibrium toward the side with less volume. In addition, pressure effect on the equilibrium is more precise than those of temperature or addition of the other chemicals. Therefore, pressure is called "thermodynamic tweezers".
Biological activity of oligomeric proteins
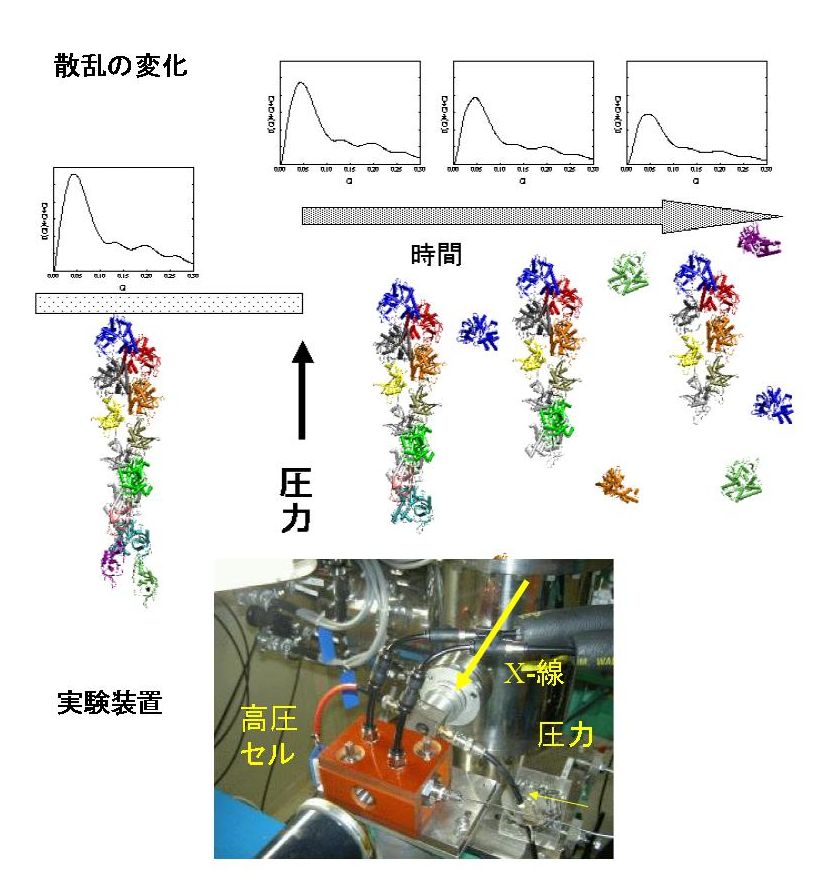
High-pressure small angle x-ray scattering
Several proteins are considered to have various conformational or association states to regulate their biological activity. Within them, most suitable states for a given condition would be selected according to the thermodynamic equilibrium. Therefore, exploring the various states of proteins over a wide range of temperature and pressure is essential to reveal the mechanism of the multi-state transition of proteins. In order to detect the conformational change or association-dissociation process of proteins, various spectroscopic methods are useful. We adopt high-pressure optical cells for small angle x-ray scattering, UV, fluorescence, and CD spectroscopy under the regulated temperature and pressure.